If you’ve done any research into shipping container homes, you know that condensation is a common concern for a lot of people. It can even go by a few names, like container rain, container sweating, wall moisture, and others. It’s a technical topic that can be confusing, but our intention is to pull back the curtain of mystery that surrounds it. We’ll teach you how condensation forms, why it is a relevant concern for shipping container homes, what the effects of condensation can be for container homes, and finally how you can stop the condensation.
It may seem a bit boring at first to learn all science about condensation, but we think it’s important for you to understand the “why” behind the “what”. There are companies out there happy to take your money for products that don’t work, and people whose well-meaning but incorrect advice will steer you in the wrong direction. So let’s start with building a solid foundation of knowledge, then we’ll jump into condensation effects and prevention.
What is condensation?
If you’re ever seen early morning dew on your grass, noticed water droplets form on the outside of a cold drinking glass, or found your bathroom mirror covered in ‘fog’ after a hot shower, you’ve experienced condensation.
Condensation refers to water in its gaseous form (known as water vapor) that changes phase into a liquid (in the form of water droplets). This phase change is caused by a temperature decrease, usually in the presence of a solid material onto which the droplets form (the grass, drinking glass, and bathroom mirror in our examples).
How and when does condensation form?
You may have noticed that in our examples of condensation above, the conditions have to be just right for condensation to form. You don’t always have dew on your grass in the morning, for example.
But what are these conditions? Hold onto your hat, it’s about to get technical! We’re going to dive into psychrometrics, which is the study of the properties of gas-vapor mixtures.
To understand condensation, we first need to understand humidity. Normally, when people talk about humidity, they’re referring to ‘relative humidity’, which is a percentage of the amount of water vapor in a volume of air compared to the maximum amount of water vapor that could be in that same volume of air at a given temperature. A relative humidity of 30% means that the air contains 30% of the moisture it could possibly hold at that temperature.
Another measure of humidity is absolute humidity, which is the mass of the water in a given volume of air at a certain temperature, often expressed as grams per cubic meter (g/m3).
As air temperature increases, the amount of water vapor that air can hold increases as well (In other words, a relative humidity of 100% will correspond to a higher absolute humidity at higher temperatures. However, if the air temperature rises but the moisture content remains the same, the relative humidity decreases). The opposite is also true: as the air temperature decreases, the amount of water vapor it can hold decreases.
It’s easier to understand with an example. Let’s say we’re at sea level and the air is saturated with water vapor (meaning it has 100% relative humidity and is unable to hold any more moisture). If the temperature is 86°F (30°C), that air will contain around 28 grams of water per cubic meter. But at a temperature of 46°F (8°C) that air will only have 8 grams of water per cubic meter (if we assume we’re still at 100% relative humidity).
Instead, let’s say that we’re again at sea level with air at 86°F (30°C), but now the relative humidity is only 50% (and therefore the absolute humidity is around 15 g/m3). As you reduce the temperature of the air, the relative humidity starts to increase above 50%, while the absolute humidity holds steady at 15 g/m3.
At some point, you’ll have dropped the temperature enough to where the relative humidity gets to 100% (while the absolute humidity will STILL be at 15g/m3). This temperature is called the dewpoint temperature and is the temperature at which the air has the maximum amount of water vapor that it can hold (a state we previously defined as being saturated). In our example, the dew point temperature is about 65°F (18°C).
What does this mean? Well, if we try to drop the air temperature below 65°F (18°C), we’ll have a problem. The lower temperature air has a reduced capacity for water vapor, but the water vapor that is already in the air needs to go somewhere. If you guessed that this excess water vapor turns into condensation, you’re right!
Staying with our example, let’s say that we want to further reduce the temperature down to 50°F (10°C). Fully saturated (100% relative humidity) air at this temperature and at sea level has an absolute humidity of around 9.5g/m3.
But remember, when we reached the dewpoint of 65°F (18°C), our cubic meter of air had 15g/m3 of water vapor. This means that to get to 50°F (10°C), 5.5 grams of water vapor will have to condense out of each cubic meter of air!
Where does the condensation physically occur? On any surface or object that is below the dewpoint temperature.
In our bathroom example, almost all surfaces of the bathroom (walls, floor, sink, mirror, etc.) take on the same temperature as the interior air of your house after hours (or days) of exposure. As the moist air from your hot shower (with near 100% relative humidity) contacts these cooler surfaces (below the dewpoint temperature), water condenses on all of them.
However, unless you take an extremely long, hot shower, you’ll probably only notice condensation on the mirror, as the water bends the light of your reflection and makes it very easy to see compared to other surfaces. Heat up your mirror and you won’t get condensation there anymore. Or, take a cold shower and you won’t have any condensation in the entire bathroom!
To further explore the relationship between air temperature, dew point temperature, and humidity, we’ve created the quick reference tables shown below. Note that the temperatures may be slightly off for altitudes above sea level.
Alternatively, you can try using several online calculators that provide even more interesting data (There may be slight differences in the results between different tables and calculators as a result of using different estimating equations).
- http://www.dpcalc.org
- https://www.ready.noaa.gov/READYmoistcal.php
- https://www.aqua-calc.com/calculate/humidity

What are the sources of moisture in a building?
It’s important to understand that condensation, usually by way of moisture-filled air, must be passed into a structure. There are a number of ways in which this can occur, some of which you may not have considered:
- Respiration (Breathing): Every time you exhale, water vapor is included in your breath. On cold days, you’ll notice the vapor condensing into fog when it encounters the cold air!
- Perspiration (Sweating): The human body’s primary cooling mechanism is the evaporation of sweat. When that sweat evaporates, it is turning into water vapor in the interior air.
- Showers: We’ve already discussed how hot showers introduce air saturated with water vapor into a bathroom. Without proper ventilation, that water vapor stays inside the building.
- Cooking: Many forms of cooking, but especially cooking that involves boiling water, are introducing water vapor into your kitchen. Similar to a bathroom, without the use of proper ventilation, this water vapor stays inside the building.
- Washing dishes: If you’ve ever opened a dishwasher right after the cleaning cycle is complete and been greeted by a face full of steam, you’ve witnessed the humidity that a dishwasher can introduce to an interior space.
- Drying clothes: While washing clothes at a very high temperature (or with a steam cycle) could introduce moisture into the interior space after opening the washer door, the more common culprit is an improperly vented clothes dryer that fails to send the hot, moist air outside the structure. If you dry your clothes naturally on a rack, the same issue applies unless you do it outside the building.
- Ironing clothes: It should be no surprise that when using the steam setting, a clothes-iron vents water vapor into the air of your space.
- Non-electric space heaters: Gas, oil-fired and propane space heaters (and even wood stoves using inadequately seasoned wood) give off moisture through the process of combustion, which can enter your space if not properly vented out through the flue.
- Damp building materials: During construction, ‘green’ wood or other materials that have been exposed to rain or other water can evaporate their moisture into the air. If you have your material closed in during part of your construction, you could end up having these damp materials trapped behind your walls where they can cause problems.
- Improper exterior sealing against liquid water: Rain, melting snow and ice, groundwater, and surface runoff all have the capacity to bring liquid water into your building if roof and wall seams and penetrations are not adequately sealed.
- Plumbing leaks: Pinholes in pipes or leaking fittings and connections can let water into your house as well, often occurring in places that are difficult to access or see.
- Pressure Differentials: When parts of your house are under negative pressure compared to the outside environment, outside air can be pulled into the structure via open doors and windows, or air leakage through smaller openings. If the outside air is warm and humid, this can introduce moisture into the structure.
It’s interesting to note that several of the highest moisture-causing activities correspond with three areas of your home: the bathroom, kitchen, and laundry areas. While outside the norm of what you’ll see in most western construction, in many countries it’s common to see all three of those located outside of the conditioned (heated and cooled) areas of the house. Doing so keeps a majority of the moisture from ever being inside the house in the first place and reduces the amount of floor area you’ll need to heat and cool.
The downside of this approach is that you’ll be cooking, bathing, and washing outside, so you might need a coat or fan, depending on where you live. It’s certainly not an option that’s appropriate for every container home project, but worth considering given that you are already breaking from the mold of typical construction by using shipping containers in the first place!
It’s clear from our list that moisture has many possible paths into and around your building. Almost all of the sources are relevant to all types of construction, not just shipping containers. So what is it about containers that makes condensation such a widely discussed issue?
Why condensation is especially important to consider with shipping container homes
Condensation is not a phenomenon that applies exclusively to shipping containers, but all metal buildings do have some properties that make condensation more of a concern than with typical construction:
- Air Leakage: Traditional construction can be prone to air leaks due to the number of individual pieces used, gaps between pieces that occur when craftsmanship is less than ideal, improper sealing around penetrations, etc. In contrast, many metal buildings (and especially shipping containers) tend to be more tightly sealed with less unintentional ventilation. While this does have some benefits like weather and pest proofing, it can also mean that if you get moist air inside, it’s less likely to leak out. Without being proactive, the moisture can be trapped indoors.
- Permeability: Permeability is the measure of the ability of a porous material to have fluids (liquids and gases) pass into and through it. Traditionally constructed buildings typically incorporate more permeable materials (such as wooden studs, sheathing, etc.) that can safely absorb (and later release, like a rechargeable battery) moisture before condensation occurs. In comparison, metal buildings are built almost exclusively out of non-permeable materials like steel (except for insulation), and thus visible condensation forms and pools more easily.
- Specific Heat Capacity: Specific heat capacity is the amount of heat energy required to increase a mass of material by one degree (or the amount of heat energy that must be lost to decrease it by one degree). As an example, for a pound of material, wood has a specific heat capacity that is about 4x greater than steel (Source). This means when exposed to a set amount of heat energy from the environment, the pound of steel will get much hotter than the pound of wood. In a metal building such as a shipping container, this means that the external temperature can have a rapid and drastic effect on the temperature of the building’s metal skin and structure. In the summer, this means heating it up quickly, but in the winter it can just as easily lose temperature and move below the dew point temperature in certain conditions.
- Thermal Conductivity: Thermal conductivity measures the speed with which heat energy moves through a material. The thermal conductivity of steel is about 300x greater than wood (Source). This means that heat can move very quickly through the steel skin and structure of a shipping container, including across any thermal bridges that exist. In the summer, these thermal bridges could cause inefficient hot spots. In the winter, the thermal bridges could cause cold spots within a container home that provide a location for condensation to form.
Since container homes are less common than traditional types of construction, some building code enforcement officials may not be familiar with the nuances of preventing container condensation. That’s somewhat a reflection of the codes themselves needing to catch up with this type of construction. Remember that what works for traditional construction may not work for container homes for the reasons stated above, so please read this article carefully.
The two types of condensation you need to understand
- Visible condensation: Moisture that condenses on surfaces you can easily see just by walking around and without tearing into walls, such as on windows, walls surfaces, exposed pipes, etc.
- Concealed condensation: Sometimes called interstitial condensation, this is moisture that migrates into and condenses inside the structure of a building in locations like wall and ceiling cavities. This type of condensation is more damaging and difficult to deal with as it is hidden behind wall coverings. Often, you don’t even know it exists until tremendous damage has already been done. There are two types of migrating moisture that cause concealed condensation:
- Diffusion (Vapor Drive): A process by which water vapor migrates through solid but permeable materials. For instance, if one side of a gypsum board (drywall) is moist and the other is dry, the moisture can make its way through the material despite it not having any visible holes.
- Infiltration (Air Leakage): A process by which air (including the water vapor contained in it) migrates through visible holes in materials and wall assemblies and gets inside the wall assembly. Example problem areas include electric switch plates, light fixtures, plumbing penetrations, and the perimeter of windows and doors.
The important thing to realize is that if you have an ongoing visible condensation problem, you most likely also have concealed condensation lurking inside your walls. If you catch concealed condensation early and give your building time to ‘dry out’, you may be ok. It’s ongoing concealed condensation that never has time to evaporate that causes problems that are difficult and expensive to fix.
The two primary conditions in which condensation can form in insulated shipping container homes
- A cold environment with interior heating: The container’s metal skin assumes the cold temperature of the exterior environment. The heated interior air can pick up moisture from the sources discussed previously. The moisture can sometimes work its way through the wall system via infiltration and diffusion. When it encounters the cold outer metal skin, it can form concealed condensation. A vapor retarder can potentially help in this situation, but that can sometimes cause more problems than it solves as we’ll discuss later!
- A warm, humid environment with interior air conditioning: The container’s metal skin assumes the warm temperature of the exterior environment. When you open doors/windows, or have an improperly sealed penetration, some warm, humid air enters the container and mixes with the interior air. If the interior of the container is kept very cool, you could have some limited visible condensation on interior surfaces that were at the cooled temperature but exposed to the warm, humid air. However, there would be little risk of concealed condensation in this case as the metal skin behind the interior wall surfaces is usually hot enough to be above the dewpoint temperature. Furthermore, the air conditioner, on top of cooling the newly introduced warm air, also reduces its humidity by allowing it to condense on the evaporator coil and exit the structure. Therefore, most of the moisture would be removed from the container automatically, and this scenario isn’t likely to occur very often.
What problems can condensation cause?
We’ve established that condensation leads to a bit of water in the interior of the building. But you may be thinking, “So what?” Well, that little bit of water can cause more issues than you may think:
- Metal damage: Rust can cause structural weakening as well as being visually unappealing.
- Masonry damage: Brick, rock, and concrete exposed to condensation and freeze-thaw cycles can lead to cracking.
- Wood damage: Condensation and moisture in the presence of wood can cause wet rot (caused by particular strains of fungi), mold, swelling, and warping.
- Coating and adhesive damage: Damage to paints, varnishes, and flooring/roofing adhesives are possible.
- Equipment damage: Condensation can lead to chemical reactions that cause corrosion in materials like fasteners, wiring, and conditioning coils. Additionally, moisture may increase the conductivity of permeable insulators in electronic devices and lead to short circuiting and other malfunctions.
- Material staining: Water spots and similar visible damage can stain building materials.
- Insulation performance: The presence of water in permeable or open-celled insulation will decrease its R-value because of the high thermal conductivity of water.
- Slip hazards: Larger quantities of condensation that form or migrate onto floors can cause slipping hazards.
- Health concerns: Moisture and condensation can cause unpleasant smells (usually from mold growth), allergy and asthma symptoms, an overall lack of comfort and productivity, and may even be a contributing factor to Sick Building Syndrome.
To see an example of condensation damage in action, read our article about Ryann’s Ohio container home. Due to initially choosing fiberglass batt insulation, she encountered major condensation damage that required demolition and replacement. The problems of condensation are real, but luckily the solutions are simple to understand and implement.
Dealing with the moisture that leads to condensation
You can control (1) the amount of moisture coming into the structure and (2) the amount of moisture leaving:
- Controlling sources of moisture:
- Showers: Ensure proper ventilation with forced ventilation.
- Cooking: Use lids when cooking or use an exhaust hood over your stove.
- Drying clothes: Ensure your dryer vent exhausts outside the building.
- Building materials: Avoid enclosure of wet building materials during construction.
- Exterior sealing: Keep rain, melting snow and ice, groundwater, surface runoff, and humid air from leaking into your building via roof and wall penetrations.
- Plumbing leaks: Ensure there aren’t pinholes in pipes or leaking fittings and connections on any of your plumbing runs that can pool and evaporate.
- Removing interior moisture:
- Dehumidification: Use a portable electric dehumidifier to remove moisture from the air, but only if you’re in a cold environment (the dehumidifiers will raise the temperature of the air).
- Air conditioner “dry mode”: Use the dry mode setting that many window and ductless AC units have to slow the fan and remove moisture from the air without cooling it significantly if you’re at a suitable temperature with too much relative humidity.
- Ventilation: Use windows, doors, and vents to replace inside air with outside air when the outside air’s absolute humidity is lower (and hence the air is drier).
A Note on Ventilation
Energy conservation, temperature control, condensation prevention, and indoor air quality are oftentimes in conflict, but ventilation affects them all. For instance, bringing fresh air inside can be great for indoor air quality, but may substantially alter indoor temperature and humidity. Factors related to ventilation include:
- Air quality: Due to the typical ‘tightness’ of container homes, ventilation is important even if it’s not necessary for moisture control because it prevents air from getting stale (full of odors, contaminants, and containing lower oxygen levels).
- Air conditioning confusion: Despite a common misconception, the majority of air conditioners do not provide outside air as part of their operation. Instead, they filter, cool, and remove moisture from the indoor air before recirculating it back into the structure. Ventilation must instead be provided by intentional (open doors, windows, and vents) and unintentional (building envelope leaks) means.
- Ventilation rate: Ventilation can be represented by the number of air changes per hour (ACH) or the cubic feet per minute (CFM) of makeup air introduced into the space. The recommendations vary by building/room use and are governed by different codes in different geographic areas, such as ASHRAE 62.1 & 62.2, IECC R403.6, IRC R303.4 & M1507, IMC 403.1 & 403.3, etc.
- Relative humidity: Failure to provide proper ventilation can cause a cumulative increase in relative humidity over time in a sealed building, absent other techniques discussed in the section above. With ventilation, if dry air is pulled into the building from outdoors, it will dehumidify the indoor air. If humid air is pulled in, it can greatly add to the humidity load that must be removed by the air conditioner.
- Pressurization: Air, whether inside or outside the building, is constantly moving from areas of high pressure to areas of low pressure. A negatively or positively pressurized room (observable via a smoke test) can have air pushed into it or out of it, along with the water vapor contained in the air. With doors, windows, and vents closed, air will try to flow through any wall penetrations and may end up inside the wall envelope.
What role do vapor retarders and barriers play in condensation control?
Vapor retarders are materials that slow the diffusion and infiltration of moisture through a wall system. Vapor barriers are just one type of vapor retarder, shown below. Vapor retarders are rated according to their measured permeance in ‘perms’. The higher the number of perms, the more vapor can pass through the material. Therefore, lower perms mean a better block against vapor.
Vapor retarders are categorized into three classes by the International Building Code (IBC), with examples of materials in each class given below (source, source, source, source):
- Class I (0.1 perm or less): Vapor impermeable
- Note: Class I vapor retarders are also known as “Vapor barriers”
- Examples: plastic polyethylene sheet, unperforated aluminum foil, sheet metal, glass
- Class II (0.1 – 1.0 perms): Vapor semi-impermeable
- Examples: kraft-facing (as on fiberglass insulation bats), exterior 1/4″ plywood, 2″ closed-cell polyurethane spray foam, vinyl wall coverings
- Class III (1.0 – 10 perms): Vapor semi-permeable
- Examples: normal latex or enamel paint, 2″ open-cell polyurethane spray foam
- Unclassified (10 perms or more): Vapor permeable
- Examples: 1/2″ gypsum board (drywall), 3.5″ unfaced fiberglass insulation bats, 3.5″ mineral (rock) wool insulation
Vapor retarders are supposed to prevent wall assemblies from getting wet. However, as an undesirable side effect, they can also prevent wall assemblies from drying effectively by trapping moisture. This is why their proper application is so important.
The Impact of Climate on Vapor Retarders
Initially, they were mostly used in cold climates, but now see increased usage (often, erroneously) in warmer environments. Used incorrectly, vapor retarders can actually lead to an increase in moisture-related problems, exactly the opposite of what’s intended.
In a cold environment, vapor retarders are typically used on the inside (warm side) of a wall assembly (normally in between the drywall and the insulation) to keep the insulation and other wall materials from being exposed to the warmer, more humid inside air which could otherwise condense inside the wall. This works fairly well for these cold climates.
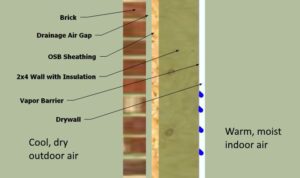
However, if used in this same manner but in a warm and humid environment, moisture would migrate through the wall system from the outside in, then encounter the cool vapor retarder (because it is close to the cold interior air) and condense inside the wall.
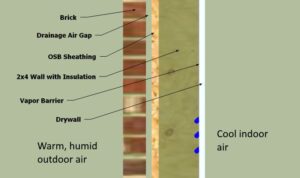
Therefore, in warm, humid climates, it’s sometimes better to either have the vapor retarder on the outside portion of the wall system or to not have any vapor retarder at all. In fact, Section 1404.3.1 of the 2018 IBC prohibits using a class I vapor retarder (and in some cases, even a class II) on the interior side of a wall system for areas in the southern United States (Climate Zones 1-4, excluding Marine 4).
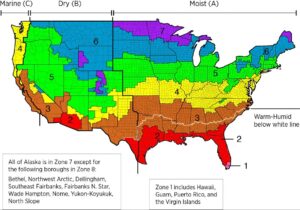
This may sound a little conflicting if you live in a place that is hot and humid during some parts of the year, and cold at other times. The fact is, you’re asking a material to do different things at different times of the year, and that’s not very realistic. Stick with us though, and we’ll give recommendations on what to do later in the article!
Now that you understand the vapor retarder dilemma for traditional construction, let’s dive a little deeper and look at vapor retarders through the lens of shipping container homes.
Vapor Retarders in Shipping Container Homes
Remember that we said that the most common container home situation in which condensation will form is with a heated interior and a cold outdoor environment, so we’ll focus there. Previously mentioned sources of moisture can make that warm interior into a warm and moist interior.
The above recommendation for vapor retarders in traditional construction in a cold environment fails to account for the fact that with container construction, the container itself is also a very effective vapor retarder. However, the vapor retarder formed by the container is located on the outside of the wall system, the opposite of the recommendation!
Therefore, placing a vapor retarder on the warm side of the interior wall, as commonly recommended, actually encapsulates the insulation between two vapor retarders. When moist air finds its way into the wall system (and it eventually will, as a perfect vapor barrier is almost impossible to construct), it can condense on the cold metal walls of the container, then diffuse into the insulation if it is permeable. Surrounded by vapor barriers on both sides, it will be very difficult for the condensation to evaporate and the insulation to dry. More than likely, problems in the wall system will result as discussed previously.
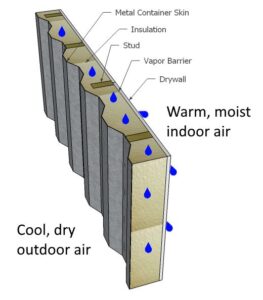
If this seems like a lot of bad news, fear not! There are several ways we can deal with condensation given the constraints we face from shipping containers.
Recommended approaches to shipping container condensation
- Concealed condensation: When condensation does occur, try to keep it from being concealed condensation. We don’t want humid air entering the wall space, whether humid air from outside, or from inside, depending on where you live and the season. You want to keep the wall/ceiling cavity airtight so any warm humid air that enters the envelope does so into the interior space and causes only visible condensation.
- Preventing diffusion into wall and ceiling cavities
- Preventing infiltration into walls by being careful when installing wiring, plumbing, windows, doors etc. and sealing around wall penetrations well
- Using insulation that is resistant to moisture movement and impregnation
- Visible condensation: If visible condensation persists, you can wipe it off with a towel, but if it returns, you really need to figure out why and how you can fix it.
- Dewpoint temperature: Ultimately, condensation of any type can only form if you have surfaces in the building envelope that are below the dewpoint temperature. The AC should quickly lower the RH and the visible condensation will evaporate. It’s a little confusing because insulation is also needed for temperature control.
- Windows: Use premium insulated windows to help keep glass temperatures above the dewpoint temperature (in warm, moist environments, the condensation can actually appear on the outside of the window, strangely enough)
- Thermal bridging: Keep anything (especially metal) in the interior of your structure from touching the exterior or metal frame of your container. Use thermal ‘breaks’ where possible, which is an insulating material placed between two pieces of metal that will slow the conduction of heat. Ensure there is insulation completely surrounding the thermal bridging item and keeping it from contacting the inside air
Note on container condensation in cold and mixed-climate environments
- Closed-cell insulation: Closed Cell Spray Polyurethane Foam (ccSPF) is what we recommend for almost all situations, and it is especially good for colder environments. Once open cell foam or other porous insulation materials are exposed to moisture, they are hard to dry and become a breeding ground for mold, etc. Closed cell foam serves as both insulation and a vapor retarder, keeping moisture out of your wall cavity. Unlike a plastic film vapor retarder, the ccSPF isn’t easily damaged, punctured, or cut and it retains the integrity of its protection. Additionally, the spray-in application fills up all the gaps in corrugation, around outlets, etc. to form a good seal. While it’s a more expensive option, we think it’s a worthwhile investment.
- Exterior insulation: Placing wall insulation on the exterior of the container is a less common option, as many people want their building to assume the shipping container aesthetic. However, exterior insulation does have some great benefits, such as an increase in interior space and lessening the chance of condensation inside the interior wall cavity. It’s also not as important to use expensive ccSPF since you aren’t space-constrained, and permeable insulation has the ability to dry out from the outside in. If you do insulate the outside, you’ll need to cover the insulation with some type of cladding to protect it from the elements and provide a more visually appealing appearance. Wood or vinyl siding, cement board, stucco, or even corrugated metal are common choices.
Note on air conditioner short cycling
- We discussed earlier how air conditioners have the ability to not only cool the air (removal of sensible heat) but also to remove moisture (removal of latent heat) and reduce the humidity. However, these processes can be greatly affected by the sizing of your air conditioner unit.
- Air conditioners remove moisture from the air by allowing the cooling coil in the interior of the building, called the condenser, to cool down to below the dew point temperature. As a fan blows the moist interior air over the condenser, water vapor condenses on the coil and slowly drips away down a condensate line where it exits the building envelope. You’ve probably seen these dripping outside.
- Each time the air conditioner turns on, it spends several minutes of operation in a dry coil condition before the condenser is cold enough to have water vapor condense on it. Note however that air cooling can still take place before this temperature is reached if the coil is below the room temperature but above the dewpoint.
- An undersized system will run continuously and never get your space to the desired temperature. That is obviously bad. An oversized system will have short run times throughout the day, and spend more of its overall daily operating time in the dry coil phase before the condenser is cold enough to remove water vapor from the air. This causes three problems. First, your air will have more humidity than you want. Second, your equipment will wear down faster, as the most taxing time of operation is during startup and shut down. Third, you’ll have paid more up front for the oversized system.
- Curious if your current air conditioner was sized correctly? On a hot afternoon, with the thermostat set at your normal temperature, time how long your system runs. If it’s less than 10 minutes (or it is coming on more than three times per hour), yet the interior temperature is ok despite high interior relative humidity, you probably have an oversized system.
How can I test my container for potential condensation indicators?
- You need to know the temperature, relative humidity, and dewpoint of the inside and outside air to make any conclusive judgments about condensation. Indoor temperature is a personal preference, but indoor relative humidity should generally be between 30-60%
- You can get a good estimate of the outside conditions by finding a weather station close to you at Weather Underground, but the further away the data is collected, the less accurate it is
- It’s better to find out the actual conditions at your location with a weather monitor of your own that can measure temperature and relative humidity, then use a calculator or table to find the dew point
- A digital thermometer/hygrometer like this one can measure indoor and outdoor humidity and temperature with the base unit plus a wireless measurement unit for the outdoors:
- Another choice is a portable unit that can measure temperature and humidity anywhere that you carry it:
- If you know the indoor dewpoint and are concerned that some of the surfaces in your building may be colder and prone to condensation, an infrared laser thermometer like this can be very helpful:
- If you’re also concerned about the CO2 levels in your building due to a perceived lack of ventilation, a desktop thermometer/hygrometer that also includes CO2 monitoring is a sound investment
Conclusion
We hope you now understand not only what condensation is, but more importantly, what you can do about it. With the right understanding of the science, it’s relatively easy to control the conditions under which condensation will and will not form. We hope this article helps you to design and live in a container home free of condensation!
Questions about the origin of condensation? Thoughts on different techniques to handle condensation? Let us know in the comments below!








31 Responses
I’m unclear on my options after reading the article. I have a 25 foot metal container used for storage of household goods. I live in the south in region 3. Mild wet winters and warm humid summers. I already have a visible mold issue after 2 years of storage. I do not have electric run to the container.
It is in a shaded location. Can you give me a best case scenario solution. The exterior is painted with a latex paint, no vents. My thoughts were the plastic barrier on the interior or a paint and moisture Large silica bags. Am I trapping the moisture inside by putting a barrier on the inside?
Thank you so much for your help!
If your container doesn’t have any vents, adding a plastic sheeting barrier to the interior isn’t going to accomplish anything. The container is already watertight as-is, and any moisture contained in the items you place inside can evaporate into the interior air and later condense on cold surfaces. If you don’t have electricity, using desiccant like silica is probably the best solution to capture the moisture before it condenses on the walls of the container.
Thanks for a great article. I’m in Tasmania (cold wet winters) and have a container under a skillion, with a glass door & window & a wood heater. Have lived in it previously, going to insulate & line it properly so it can be used as living quarters & also storage without mold issues. Caveat is I don’t have much money. So my current plan is this: frame out internally with timber, fill cavity with glasswool insulation pressed against steel like a roof blanket e.g. Anticon, then line with 12mm plywood. But first seal the plywood both sides with polyurethane. So will the PU on the ply act as a vapour barrier, or should I also install a plastic barrier first? Should I go around with a PU caulking gun & seal every join & hole? Dip screws in it before fixing off the ply, or is that excessive? Seal off the 2 external vents to prevent outside humidity from entering wall cavity? I do take your point about the closed cell insulation, but for the money I’d be better off selling the container & just building a cabin that breathes properly.
We’ve never heard of anyone doing this, but theoretically, it should work. Basically, you’ll have to completely seal every penetration so that the cavity between the plywood and the container is completely isolated from the indoor and outdoor air (easier said than done, which is probably why no one undertakes this approach). You’d also need to read the manufacturer specifications for the polyurethane…not sure how permeable it is. If all of that checks out, you shouldn’t need additional plastic sheeting.
Thanks for such a fast response. If I can push it a bit further, is my choice of insulation or lining that is not done, or simply my climate zone?
Condensation is the absolute priority – comfort is barely a factor – so would I be better off leaving the walls bare & simply using a lot of dessicant?
Thanks again for your time.
It’s hard to know the best solution without being on-site and looking at temperature, humidity, shade, etc. as well as understanding your budget, planned usage, etc. We don’t typically recommend using a desiccant for a container that will be used for habitation just because of the volume of moisture you’ll likely generate. At the end of the day, just remember the most important part of the article: condensation can only form if you have surfaces that are below the dew-point temperature. There are a number of ways to ensure that doesn’t happen, and how best to achieve it for your specific situation is something you’ll have to think about a bit. If you have further questions, feel free to email us via the Contact Us page.
I have a 40′ container. I have a moisture problem on the inside. I just store different thing on the inside. Its still just like it came from manufacturer. I was thinking about putting vent in it two on one end on the doors and two on the other end on the side walls. I was going to put two on the bottom of the doors and two up high on the side wall on the other end, Whats your thoughts on this????
Thanks
Mac
Ventilation may help, but if there is still a large difference between the air temperature and the container’s metal skin, condensation could still be an issue. In the case of using one for storage (where powered devices like dehumidifiers and air conditioners aren’t practical), a desiccant pack is something to look into.
looking to install a sauna in a shipping container. The plan is to insulate with closed cell spray foam on the interior as well as use rigid insulation on the exterior. Climate would be mild to cold and am a bit worried about the extreme temperature differential causing condensation becoming trapped between the spray foam and shipping container. Is it just a matter of adding enough insulation on the exterior so that the dew point is on the exterior of the container?
Well, you first need to decide if it’s going to be a wet sauna (steam room) or dry sauna, meaning, are you going to artificially introduce even more moisture into the interior over and above what will result from the perspiration of the occupants. Secondly, if you’re going to insulate the exterior, think through the purpose of insulating inside as well. If your exterior insulation can keep the container above the dew point temperature, then condensation shouldn’t occur on the interior of the container, and interior insulation isn’t really needed. However, if you have a high humidity environment inside the container, even if there is no condensation, just the high humidity in the air could lead to corrosion. Therefore, you’d want to ensure you have a good paint/coating on the interior.
My daughter’s bone chamber is made up of 2 sides cement and 2 sides glass. For a year it did not have inside mist/dew in the morning…after a year the mist began to form every morning on the glass side exposed to sun and rain though it disappears later in the day….but every morning there is mist…and for sometime now it has stain….Please help me what to do as the glass is sealed with sealant….thank you very much I hope you can help me solve this problem…by the way my daughter’s bone chamber is outdoor, exposed to sunlight and rain…thank you again…
We aren’t familiar with bone chambers, but the principles of heat transfer and condensation apply universally. It sounds like it may a leak that let some moisture inside – in the morning it’s cool enough that the moisture condenses on the glass, but later in the day the air inside heats up enough to vaporize the moisture. If you can’t vent it out, you might try using the silica gel packs that are commonly provided in new shoes…they absorb moisture.
Thanks a LOT for this, very educational!
Thanks for this great article and one that I really should have read before I fitted out my 20ft high-top container. Be that as it may, I built the container and fitted it with 50mm (2′) sandwich freezer wall panels, 3 windows and doors. The seal of the freezer panels is sealed with Sycaflex and the all the window trims are plate steel. We live in Oz at 34 degrees south. Wet winters. Dry summers. Condensation is a problem on the windows and the plate steel window sills in winter. I run a small de-humidifier to combat this. . . . but it’s not enough. From reading your article, my two solutions are either to install a small split-cycle AC (too expensive to run IMO) or to externally insulate and clad. Are there any other retro-fit solutions that I’ve missed? Thanks again for this article. . . makes perfect sense. Now. Better late than never.
Glad you enjoyed the article, and sorry you didn’t find it in time! Sounds like you may have thermal bridging through the window trims, depending on how they are constructed. It might help to have a thermal break if there is continuous metal going from outside to inside around the window. The other thing is if you aren’t using insulated (like double-paned) windows, that could also be contributing to the problem. You could also upsize the ‘small’ dehumidifier you’re using…we’d recommend measuring the humidity inside to see how effective it is.
The split unit AC will basically do the same thing the humidifier is doing. External insulation will help, but if you’ve already insulated on the inside, tweaks (like what we mentioned above) to what you already have should work instead.
if i was to glue and screw jib to the inside walls of the reefer container, without a cavity would the be a risk of condensation and would it damage the jib.
my intention is to clad the outside of the reefer container with ACM or Aluminium weather board with a cavity.
Not sure we completely understand the question, though it may partially be an issue of terminology, as we’re not sure what you mean by “jib” unless some kind of horizontal runner, but that doesn’t really make sense given the context. Perhaps sending us an email via the ‘contact us’ page and sharing a little more detail would be best and we can discuss your specific question there.
I am actually from Belgium and looking into building a home with shipping containers. Insulation standards are probably different here, but the idea is the same. Unlike most of you, I am considering exterior insulation with finished panels. Single digit winters and 100 degree summers with usually low humidity. Will the exterior insulation suffice or do in need to insulate the interior with closed cell PUR foam behind the drywall sheet? Bathroom and open kitchen will be ventilated. I am mostly concerned about thermal bridges near the roof.
Jonas, if you’re happy using external insulation, why are wanting to use drywall inside? With external insulation, you can leave the container walls exposed to the interior, which a lot of people prefer. If you’re going to cover up both the interior and exterior of the containers, perhaps containers aren’t the best choice for your application?
As far as thermal bridges, if you completely surround the outside of the container, thermal bridges should be minimal. You’ll be controlling any penetrations in the insulation you make, which is where thermal bridges might occur. Winter condensation on the interior should be eliminated if you keep the container externally insulated so that the container skin doesn’t drop below the dew point temperature.
Hello, I am considering converting two/three one trippers into a contain home in St. Louis, MO. I read through your article and really appreciate the insight into the condensation conversation.
I am wondering what your advice would be on insulating a container home in our midwest climate would be? Our summer temps go as high as 100 degrees with lots of humidty, and our winter temps dip well into the single digits. So there will definitely be a need for air conditioner and heating…
I am slightly confused on what to do with this type of drastic outdoor temperature throughout the year.
Any help would be awesome. Thank you.
When you have very low winter temperatures part of the year, we recommend using closed-cell polyurethane spray foam (assuming you’re insulating inside the container). In the summer, your air conditioner will be dehumidifying the room automatically, so no big deal there. It’s the winter time where the condensation becomes more of a concern, and using ccSPF will seal up the wall cavity so it’s nice and dry, as well as giving you a high R-value per inch. This will help ensure that if you do happen to have any condensation, it should be visible. You’ll notice it right away and you can increase your ventilation.
I live in western New York, so a cold environment where an interior heat source would be required. I’m in the early phase of planning to convert a 40′ container into a guest house/rental house, so peak usage would be May-October. But I’d like to plan the design so that it would be usable year-round. Even in the peak months the temperature can be cool enough to require a bit of indoor heat. The container will almost certainly be off-grid, with electricity only through solar panels and battery storage. So the interior heat would ideally be wood heat with a high-efficiency wood stove. These stoves significantly dehumidify the indoor air.
Given all that, am I correct in inferring that an ideal recommendation for our situation would be closed-cell spray insulation and no interior vapor barrier? Possibly an additional dehumidifier if needed? Should I attempt to find climate data on days where conditions reach the dew point?
I’m attempting to calculate the electrical load so I can budget for an appropriately-sized solar array.
Thanks for the in-depth articles, and any guidance on my above query.
Yes, we recommend using polyurethane closed-cell spray foam, and not using any additional vapor retarding material in the wall. Depending on your sources of moisture, you may have to consider a dehumidifier. If the guest house will have a bathroom inside, that will usually be your biggest source of moisture, and good ventilation there will be important. HVAC professionals use ‘degree days’ to figure out how to size units, so you could consider using a calculator like https://www.degreedays.net/ for this, although how to actually interpret and use that data is a whole other set of questions. Weather is obviously always changing and hard to predict. It’s quite possible that although on the average you may have a cold and dry environment, there may be a few days where it’s hot and humid. You can’t design your home/systems for every possible scenario, just the averages. On the days with crazy weather, you may need that portable dehumidifier, or to open the windows, etc. Hope that helps!
So, in a container home in the middle of the jungle in Yucatan, Mexico, where it is always hot 75 to 100 oF and always humid 70 to 100 % what is the basic recommendation?
We definitely use AC profusely, through our own solar generated electricity.
To insulate the tops I’m thinking of a 6 – 8” soil and grass ‘roof garden” does that sound like a good idea?
The benefit of an area where it is always hot is that interior condensation won’t really be an issue, as long as you’re using AC. As far as insulation goes, you obviously have quite a few options. However, soil is not a good insulator. Our article on heat transfer (https://www.discovercontainers.com/essential-knowledge-about-heat-transfer-in-shipping-container-buildings/) includes a link to a website with R-values for commmon materials, which will give you an idea for how soil compares. If you want to keep heat out, we’d recommend a better insulating material. However, if you don’t mind running your AC more, then insulation isn’t as much of a concern. As the article suggests, a major reason for insulating is keeping condensation controlled, which isn’t really an issue for you. The other thing to think about with soil on top of your container is weight. The roof is not designed to support hardly any load, and you’ll need to make some reinforcements if you’re planning on adding all that soil. In addition, you’ll need a good waterproof coating and drainage to prevent wet soil from rusting out your container roof!
Great article guys, This is not common knowledge for most people. As a result most never consider it until it’s a problem. A stick-built house with vinyl siding doesn’t really experience these issues too much.
And oftentimes, you don’t know it’s a problem until you already have damage! Thanks for the kind words, we’re glad you enjoyed the article and found it helpful.
I have 3 shipping containers I have used to build a hunting cabin out of and close it up when not there. I use 2 fans I use to keep the air inside circulating to help with the inside moisture. Seems to control the moisture inside. I use a humidity/temperature monitor to check on the humidity while I’m not there. Is this a logical way to help control the moisture?? Thanks jimmy
If by ‘circulating’ you mean exchanging inside and outside air with ventilation, that’s good. If you’re just moving inside air from room to room, that isn’t going to help your overall interior humidity level. The temperature/humidity monitor tells the whole story though, and if you can keep an adequate interior temperature and humidity, then keep doing what you’re doing. A note for others who are reading this…It sounds like you are using this building sporadically, which may be giving your humidity a chance to ‘bleed off’ via leakage before your next use. That’s to say if you were continuously occupying the building, you *might* find that your current method of condensation control is insufficient, though it may work for your current limited usage.
I found the topic and explanation excellent but I would like to offer an added consideration for those who periodically have condensation problems.
Im working to get totally Off Grid and one items of our household is a Dehumidifier. Here in the southern part of New Zealand, we don’t need air conditioning and more and more folks are getting sucked in by all the hype of Heat Pumps.
We are quite happy with using a cosy fire as heating and leave the Dehumidifier in the bathroom where it takes the high humidity out of the air.
This of course reduces the excessive needs of a roaring fire, as its the moisture in the air which is what needs to be warmed. If there aint no water, then a much less amount of heat required to get a room /house warm.
If there is one item which I REALLLLLLLY want to operate with in ANY building we construct or build or alter, its a good, quiet Dehumidifier. ( We find that Mitsubishi is ideal and is both quiet, effective and efficient in $ terms, to operate.)
I’m planning to get solar panels etc, so as it can operate Off Grid.
I am converting a 40 ft Insulated shipping container (aka a Reefer) and the main reason I chose an Insulated Container, rather than just a bare steel box type, is because of the condensation issue and also ’cause I want a premium grade of insulation.
When the conversion is complete, I will put a video up on Youtube, of the methods and ways of what we want in it and how it was done.
It having a Kitchen with a Rayburn cooker, (with a couple of cast iron radiators for heating) Shower, storage room, composting Dunny (WC) & Bidet.
Its 1st Sept 18 today and it will probably take me more than another 3 or 4 months before its on Youtube. I’m being fussy and doing stuff I ain’t done before, so it may take another year before its totally complete.
TTFN
(as Winnie the Poo says.)
Humidifiers are an option worth looking into if you’re in a cooler climate where heating is your primary means of climate control. Heat pumps have their place, but they are certainly more expensive if a humidifier and a heater will do the job. Be careful with the fire though, as mentioned in the article, it can be releasing a lot of water vapor into the air.